What we can do to help you have a safe birth?
While you are excited to see your baby, we know that some expecting mothers are worried about their baby.
In order to eliminate such worries, knowing the health of the fetus in advance through a prenatal diagnosis will make it easier for you to prepare to welcome your incoming baby.
HUMEDIT NIPT provides NIPT (Non-invasive prenatal testing) that is safe for both mother and fetus. By having the test performed by the ‘Tokyo Clinical Laboratory’, a domestic specialist laboratory for human genetic testing, the results can be obtained more quickly and accurately than with the conventional NIPT, which was performed by an overseas specialist laboratory. Results are usually delivered within 10 days after the blood sample is taken. The VeriSeqNIPT Solution V2 system used at the Tokyo Clinical Laboratory has been widely used for clinical testing in Europe and the United States, but HUMEDIT NIPT is the first clinic in Japan to implement this system. As of November 2022, the number of tests performed in Japan has exceeded 26,000 cases, and the number of tests performed in Japan and overseas has exceeded 28,000 cases. We have accumulated a large number of cases and share the analysis results with the Tokyo Clinical Laboratory to improve our testing.
We are proud to say that HUMEDIT NIPT is the only clinic in Japan that can test for chromosomes other than chromosome 21, 18, and 13 Trisomy and has tested a large number of cases. HUMEDIT NIPT offers NIPT testing at a total of 10 clinics from Sapporo to Fukuoka. Each clinic is staffed by specialists in obstetrics and gynecology, clinical genetics, pediatrics, and psychiatry. Each clinic is connected to each other through a network, which makes it possible for each specialist to answer various questions from expectant mothers.
One-dimensional barcodes issued by HUMEDIT are seemlessly read by the Tokyo Clinical Laboratory’s testing equipment (Hamilton MicroLabstar), so there is virtually no risk of false readings.
One of the great assets of HUMEDIT NIPT is that you can undergo testing for sex chromosomes and total autosomal aneuploidy at an affordable price.
At HUMEDIT NIPT, we offer a variety of Test Set Plans so that we can provide information that expectant mothers and their families wish to know and deliver it in an easy-to-understand manner.
Over 28,000 NIPT tests performed on pregnant women across Japan
In NIPT studies around the world, it is known that genetic disorders have both ethnic and regional characteristics.
However, despite the availability of various research data, mainly from Europe and the U.S. but also from Asia, there have been no reports of genetic disease data specifically on pregnant women in Japan.
HUMEDIT NIPT has analyzed data from over 28,000 patients* who have undergone NIPT in cooperation
with Second Tokyo Clinical Laboratory. (*As of November 2022)
Based on these data, we propose test items that are truly necessary for pregnant women in Japan. HUMEDIT NIPT believes that analyzing and reporting as much data as possible with the current technology protects the “right to know” of pregnant women.
Testing is available throughout Japan
There are now 10 HUMEDIT NIPT clinics operating from Hokkaido to Hakata, and testing is also available at 14 partner clinics throughout Japan.
Positivity Score Report, an analysis of data from 10,000 pregnant women in Japan
If you get a positive result, you will know how positive it really is. Based on the results of over 10,000 NIPT tests performed at HUMEDIT NIPT and the numerical analysis of amniotic fluid test results, we offer a free Positivity Score Report that provides a combination of a “Positivity Score” and “Positivity Predictive Rate”.
Twins can also be tested

Medical facilities affiliated with the NIPT Consortium do not offer NIPT testing for twins, and medical facilities outside of the NIPT Consortium only offer NIPT testing for twins with chromosomes 13, 18, and 21. HUMEDIT NIPT can test All chromosomes for chromosome abnormality and All autosomal partial deletions and duplications in twins. However, regarding sex chromosomes, only the presence or absence of the Y chromosome can be tested at this time.
Consult with a specialist of the Japanese Society of Obstetrics and Gynecology

HUMEDIT NIPT Tokyo, Nagoya and Hakata are staffed by physicians specializing in obstetrics and gynecology from the Japanese Society of Obstetrics and Gynecology.
At our Tokyo, Nagoya and Hakata Clinic, fetal ultrasound examinations can be performed prior to the NIPT examination. Having a thorough knowledge of the condition of the fetus before undergoing the NIPT examination gives patients a peace of mind.
Details here
Difference between accredited and non-accredited facilities
for NIPT (new non-invasive prenatal testing)
There are two types of facilities that can perform NIPT testing: Accredited and Non-accredited
What is an Accredited Facility?
Accredited facilities are NIPT facilities accredited by the Japan Federation of Medical Societies.
The accreditation of a facility also imposes requirements on patients who undergo NIPT testing.
- when the fetus has a positive result for chromosomal abnormality on fetal ultrasound examination
- when the fetus has a positive result for chromosomal abnormality on maternal serum marker test
- if the mother has previously given birth to a child with a chromosomal abnormality
- both parents have a balanced Robertson translocation and there is a possibility of inherited chromosome abnormality
In addition to these requirements, there are other hurdles, such as having to be at least 35 years old and needing a referral letter from an obstetrician/gynecologist.
We report not only on trisomy, but also on monosomy
HUMEDIT NIPT reports not only trisomies (three chromosomes) but also monosomies (one chromosome) and other abnormalities on chromosomes 21, 18, 13, and all chromosomes.
All chromosome testing
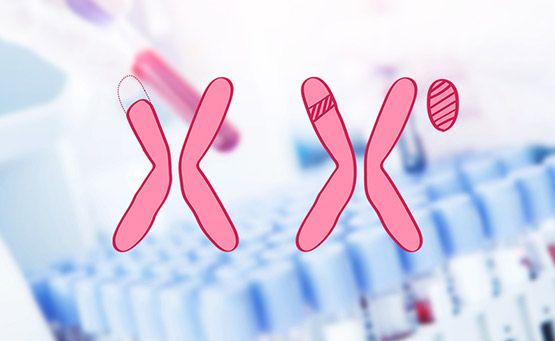
While the conventional NIPT examines for 21, 18, and 13 trisomies, the all-chromosome test examines for abnormalities in chromosomes 1 through 22 and in the number of sex chromosomes.
All autosomal whole region partial deletion/duplication disease
Although some facilities other than those in the NIPT Consortium can test for five types of partial deletion diseases (1p36 deletion syndrome, Wolff-Hirschhorn syndrome, Cri-du-chat syndrome, Prader-Willi syndrome, Angelman syndrome, and Di George syndrome), HUMEDIT NIPT is the only facility in Japan* that can test for more than 54 types of all autosomal partial deletions and duplications. However, the minimum size that can be detected is 7 million bases. Deletions and duplications smaller than this are undetectable, so it is not possible to detect all whole autosomal partial deletions. If there is a partial deletion in a location that is reported as an all-autosomal whole region partial deletion disease, the test report is generated as that all-autosomal whole region partial deletion disease. * As of October 2021
Cost of NIPT
Testing fee for NIPT
The cost of the test is approximately Php27,000 – Php50,000, depending on the test procedure. Most facilities accredited by the Japan Medical Association include the cost of the definitive test in the price of the new Non-Invasive Prenatal Testing (NIPT).
HUMEDIT NIPT Testing Fees
Minimum Plan – an affordable plan that includes testing for trisomy 21, 18, and 13 and monosomies, including Down syndrome, and costs ₱27,000 (tax exclusive).
Light Plan – includes sex chromosome (number abnormality) testing in addition to the Minimum Plan, and costs ₱30,000 (tax exclusive).
Full Set Plan – includes testing for all trisomies and monosomies on chromosomes 1 to 22, plus testing for partial deletions and duplications on chromosomes 1, 2, 3, 4, 5, 7, 8, 10, 15, 18, 20, 21, and 22, for ₱50,000 (tax exclusive).
HUMEDIT NIPT Testing Policy
At HUMEDIT NIPT, there are no age restrictions on testing.
The following is a summary of the American College of Obstetricians and Gynecologists’ guidelines for NIPT.
Summary of Society Guidelines on NIPT
-
All women should be offered the option of aneuploidy screening,
including NIPT, or diagnostic testing regardless of age.1- 3 -
If NIPT fails to give a result, alternate testing should be discussed
with the patient because of increased risk for aneuploidy. 1-2 -
False positives and false negatives do occur. Diagnostic testing
should be offered to any patient with a positive NIPT result. 1-3
- American Colege of Obstetriciansa nd Gynecologists. Screening for fetal aneuploidy. Practice Bulletin No.1 63.O bslef Gynecot.2 016;1 27(5):e123-137.
- Benn P, Borrell A, Chiu RWK,et al. Position statement from the Chromosome Abnormality Screening Committee on behalf of theB oardo f the lnte rnational Society for Prenatal Diagnosis. Prenat Oiagn.2 015;35(8):725-734.do i:10.1002/pd.4608.
- Gregg AR,S kotkoB G, Benkendorf JL,e t al. Noninvasive prenatat screening for fetala neuploidy,2 016u pdate: ap osition statement of the American College of MedicalGe netics and Genomics.G enet Med.2 016: doi:10.1038/gim.2016.97.
When can NIPT be performed?
NIPT can be done as early as 10 weeks of pregnancy. Appointments can be made as soon as the due date is known.
Blood extraction can be completed in 30mins. Results are usually available within 10 days after blood collection at HUMEDIT NIPT.
HUMEDIT NIPT System
Overview of NIPT Test
| Test name | NIPT (Non-Invasive Prenatal Testing) |
|---|---|
| Blood collection Medical institution | HUMEDIT NIPT |
| Specimen | 10 ml of blood |
| Test Subjects | Pregnant women after 10 weeks, Single or twin pregnancies |
| Age limit | None |
NIPT Results for Low Risk Groups
| Articles | Number of pregnant women | Detection rate | False Positives |
|---|---|---|---|
| Nicolaides et al.[※1] | 2049 | 100% | 0.1% |
| Dan et al.[※2] | 11,105 | 100% | 0.03% |
| Bianchi et al.[※3] | 2052 | 100% | 0.3% |
| Norton et al.[※4] | 15,841 | 100% | 0.06% |
| Zhang et al.[※5] | 147,314 | 99.17% | 0.05% |
[※1]Nicolaides KH,Syngelaki A, Ashoor G, Birdir C,Touzet G. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. Am J Obstet Gynecol 2012;207:374.el-6.
[※2]Dan S, Wang W, Ren J, Li Y, Hu H,Zu Z, et al. Clinical application of massively parallel sequencing-based prenatal noninvasive fetal trisomy test for trisomies 21 and 18 in 11105 pregnancies with mixed risk factors. Prenat Diagn 2012;32:1225-32.
[※3]Bianchi DW, Lamar Parker R, Wentworth J, Madankumar R, Saffer C, Das AF, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med 2014;370:799-808.
[※4]Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H,et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med 2015;372:1589-97.
[※5]Zhang H, Gao Y, Jiang F, Fu M, Yuan Y, Guo Y, et al.Non-invasive prenatal testing for trisomies 21,18 and 13:clinical experience from 146958 pregnancies. Ultrasound Obstet Gynecol 2015;45:530-8.
Sensitivity and specificity of NIPT
Sensitivity and specificity in 21 trisomies, 18 and 13
N=2028 Including mosaic
| Sensitivity Two-sided 95% confidence interval | Specificity Two-sided 95% confidence interval | |
|---|---|---|
| Trisomy 21 | > 99.9% (130/130) (97.1%, 100%) | 99.90% (1982/1984) (99.63%, 99.97%) |
| Trisomy 18 | > 99.9% (41/41) (91.4%, 100%) | 99.90% (1995/1997) (99.64%, 99.97%) |
| Trisomy 13 | > 99.9% (26/26) (87.1%, 100%) | 99.90% (2000/2002) (99.64%, 99.97%) |
Estimated sensitivity and specificity for twins
21 trisomy, 18 trisomy, 13 trisomy, presence of Y
| Sensitivity Two-sided 95% confidence interval | Specificity Two-sided 95% confidence interval | |
|---|---|---|
| Trisomy 21 | 96.40% (86.4%, 98.9%) | 99.90% (99.8%, > 99.9%) |
| Trisomy 18 | 95.70% (68.3%, 99.4%) | > 99.9% (99.9%, > 99.9%) |
| Trisomy 13 | 93.60% (64.1%, 98.9%) | > 99.9% (99.9%, > 99.9%) |
| Presence of Y | > 99.9% (99.9%, > 99.9%) | > 99.9% (99.7%, > 99.9%) |
Chromosomal aneuploidy and fetal gender identity
| Sex Classification | Physical findings | Genetic Testing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Result | Girls | Boys | XX | XY | XO | XXX | XXY | XYY | Other abnormalities | Unknown | |
| Negative | XX | 997 | 0 | 21 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Negative | XY | 0 | 966 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 1 |
| Positive | XO | 0 | 0 | 0 | 0 | 19 | 0 | 0 | 1 | 0 | 0 |
| Positive | XXX | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 0 | 1 | 0 |
| Positive | XXY | 0 | 0 | 0 | 0 | 0 | 0 | 23 | 0 | 1 | 0 |
| Positive | XYY | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 |
| Total | 997 | 966 | 21 | 15 | 21 | 17 | 23 | 12 | 2 | 1 | |
Sensitivity and Specificity of Veriseq NIPT Solution V2
for Detecting Any Abnormality (including Mosaic)
| Sensitivity | Specificity | |
|---|---|---|
| Estimate %(n/N) | 95.5% (318/333) | 99.34% (1954/1967) |
| Two-sided 95% confidence interval | 92.7%, 97.3% | 98.87%, 99.61% |
Sensitivity and Specificity of All Autosomal Chromosomes (Including Mosaicism)
| Sensitivity | Specificity | |
|---|---|---|
| Estimate %(n/N) | 96.4% (27/28) | 99.80% (2001/2005) |
| Two-sided 95% confidence interval | 82.3%, 99.4% | 99.49%, 99.92% |
All Autosomal Whole Region Partial Deletion/Duplication (Including Mosaicism)
| Sensitivity | Specificity | |
|---|---|---|
| Estimate %(n/N) | 74.1% (20/27) | 99.80% (2001/2004) |
| Two-sided 95% confidence interval | 55.3%, 86.8% | 99.49%, 99.92% |
Negative Medium Rate
99.9% Sensitivity and 99.9% Specificity of Negative Predictive Value
| Age at Time of Testing | Case | Frequency | Positive Medium Rate | Negative Medium Rate |
|---|---|---|---|---|
| 49 years old | Trisomy 21 | 8% (1/13) | 98.86% | 99.99% |
| 40 years old | Trisomy 21 | 1% (1/100) | 90.983% | 100% |
| 28 years old | Trisomy 21 | 0.1% (1/1,000) | 50% | 100% |
| Trisomy 13 | 0.01% (1/10,000) | 9.08% | 100% |
For those with a low positive predictive value, we recommend an amniotic fluid test as a definitive test even if the NIPT is positive.
Differences in testing contents in different laboratories
This is a comparison table of inspection contents between HUMEDIT NIPT and other inspection institutions.
| HUMEDIT NIPT | NIPT Consortium | Medical Institutions other than NIPT Consortium | |
|---|---|---|---|
| Testing Institute | Affiliated Second Tokyo Clinical Laboratory (Bunkyo-ku, Tokyo) | Domestic Testing (GeneTech etc.) | Overseas (Verinata Healthcare etc) |
| Number of days for inspection report | Delivery is usually 10 days after the time of blood collection. | 1-2 weeks | 1-2 weeks |
| Fee | Lowest standard price in the industry | Expensive | Expensive |
Positivity Score Report
Positivity Score is an optional service of HUMEDIT NIPT. If the NIPT is positive, the Positivity Score Report will help you decide if you should have an amniotic fluid test.
| Second Tokyo Clinical Laboratory | Gene Tech | Verinata Healthcare | |
|---|---|---|---|
| Positivity Score | ○ Chromosomes 21, 18, and 13 trisomy only | × | × |


